Background: Haploidentical (haplo) transplantation using post-transplant cyclophosphamide (Cy) based GVHD prophylaxis is a well-established alternative donor approach for allogeneic hematopoietic cell transplantation (HCT). Although outcomes from haplo HCT have been compared to matched related (MRD) and unrelated donor (MUD) allo HCT, quality-of-life (QOL) recovery between these transplant modalities has not been described. We hypothesized that post-transplant QOL is similar between Haplo and MRD/MUD grafts. A single-institution, retrospective cohort study was therefore conducted to compare outcomes including QOL between these groups.
Methods: From May 2014 through December 2019, 90 haplo, 102 MRD and 229 MUD adult 1st allo HCTs were performed. Myeloablative conditioning (MAC) for haplo grafts (n=35) included fludarabine (Flu)/ total body irradiation (TBI) and reduced-intensity conditioning (RIC) haplo transplants (n=55) included Flu/Cy/TBI. QOL was assessed prior to transplant and at days +100 and +180 by the FACT-BMT which consisted of the following subdomains: "Physical well-being," "Social well-being," "Emotional well-being," "Functional well-being," "Additional concerns," and sum of all these subdomains as "Total score." Outcomes were estimated and compared with the Kaplan-Meier and log-rank test or cumulative incidence and Gray test. FACT-BMT scores were compared with repeated measures analysis of variance.
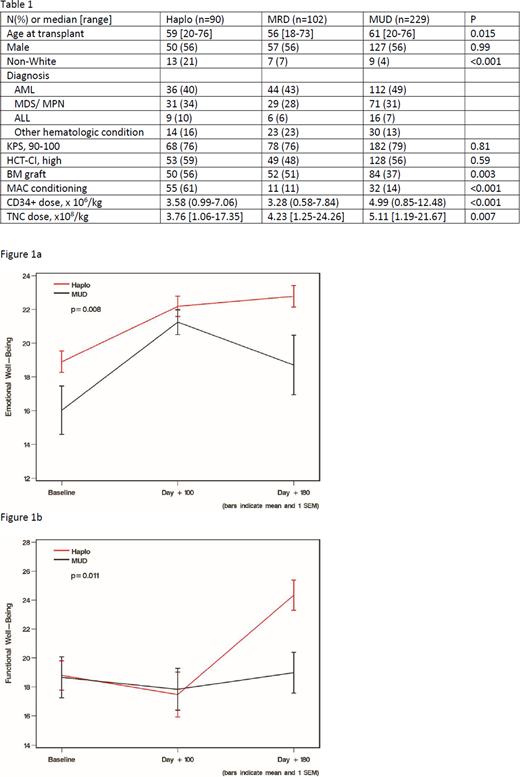
Results: Patient and transplant characteristics were comparable among the donor sources except for higher median age at HCT in MUD transplants, and haplo grafts were more commonly used non-Caucasians, used RIC regimens and bone marrow grafts (Table 1). Median transplant hospital length of stay was longer for haplo vs. MRD vs. MUD grafts (31 vs.25 vs. 25 days, p<0.001) with longer median time until neutrophil and platelet recovery for haplo compared to MRD and MUD grafts (20 vs. 14 vs. 15 days, p<0.001, and 31 vs. 21 vs. 22 days, p<0.001, respectively). MAC haplo HCT had a higher incidence of CMV (p=0.05) and other infections (p=0.003), but no difference in other outcomes compared to MRD/MUD grafts, including all of the QOL domains. The mean Total scores from baseline to day +180 for haplo HCT were 157-157, MRD 153-166, and MUD HCT 152-163. RIC haplo HCT was associated with higher incidences of infections (p<0.001) and relapse mortality (p=0.04), but lower NRM (p=0.008) and no differences in other outcomes. When comparing RIC haplo to MUD transplants there was significantly better Emotional well-being (p=0.008) and Functional well-being (p=0.011) at day+180; respective mean emotional well-being score for haplo 19-23 and MUD 16-19; mean functional well-being score for haplo 19-24 and MRD 19-19 (Figures 1a and 1b). No difference for the other QOL measures were found; the mean Total scores from baseline to day +180 for haplo HCT were 155-180, and MUD HCT were 150-158 (p=0.25).
Discussion: Among MAC recipients, we observed no differences in total FACT QOL scores and individual domains among haplo, MRD and MUD HCT recipients through 6 months post-HCT. After RIC HCT, haplo recipients reported better Emotional and Functional well-being compared to those receiving a MUD graft, although Total FACT score and other QOL domains were comparable through 6 months. Future strategies to lessen infectious complications and relapse may further improve QOL outcomes. Interrogation of QOL among disease specific groups may also elucidate whether additional benefits exist between these different transplant modalities.
Gerds:Sierra Oncology: Research Funding; Gilead Sciences: Research Funding; Celgene: Consultancy, Research Funding; Imago Biosciences: Research Funding; Roche/Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Apexx Oncology: Consultancy; AstraZeneca/MedImmune: Consultancy; Pfizer: Research Funding. Hamilton:Syndax Pharmaceuticals: Consultancy, Honoraria. Majhail:Nkarta Therapeutics: Honoraria; Incyte: Honoraria; Mallinckrodt: Honoraria; Anthem, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal